Toe-niquets™ are Single Use disposable 'elastic-ring' tourniquet devices designed to stop blood flow in digits of the hands and feet (fingers and toes) for use during surgery, trauma or first aid. Toe-niquets™ are easily applied by rolling over the tip of any digit.
Toe-niquets™ allow you to exanguinate (remove) blood from a digit and stop further blood flow at the point of application. Treatment may continue safely, quickly and easily without the risks of visual or physical obstructions associated with bleeding.
Toe-niquets™ are used during surgery, trauma or first aid by Medical Doctors, Surgeons, Surgical Theatres, Emergency Room Facilities and Podiatrists worldwide. They are supplied sterile ready for use in any clinical or surgical environment.
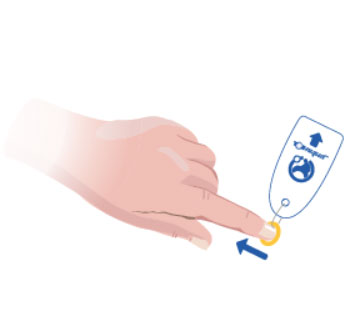
1. Prepare the patient fully ready for treatment and select the correct size Toe-niquet™.
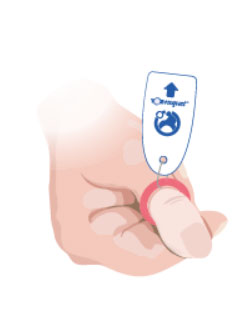
2. As the Toe-niquet™ is applied the blood is pushed out (exanguinated).
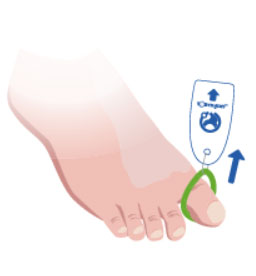
3. Apply an appropriate dressing or wound closure.
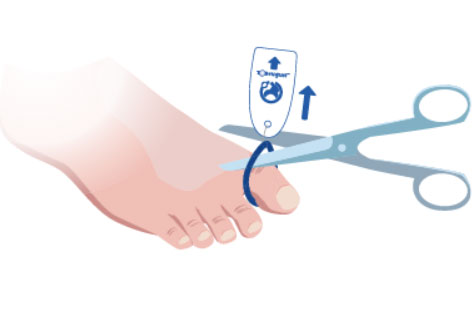
4. Pull on the Tag and nylon tie to lift the Toe-niquet™ and cut to remove.
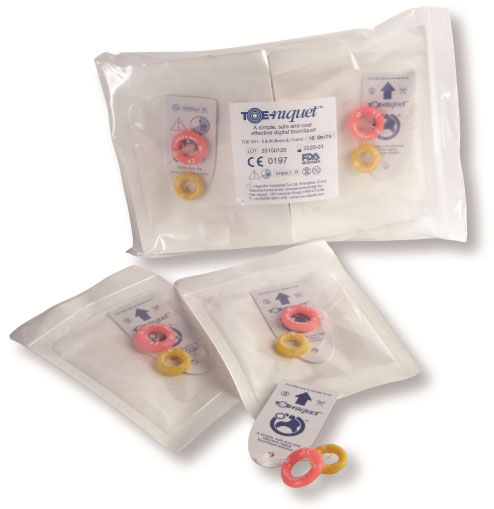
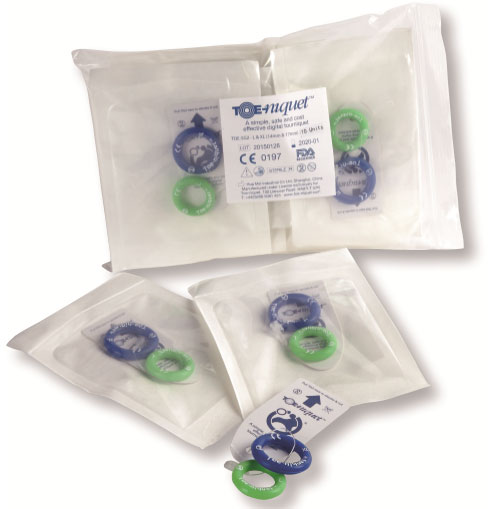
Toe-niquets™ are individually packed, sterilised by irradiation and offer a 5 year shelf life. The peel pouch is made from high quality Tyvek and conforms to BSEN-5 1999 Standard, providing full traceability with batch numbers for medical records.
Toe-niquets™ carry the 'Single Use'’ only symbol for improved patient safety.
Toe-niquets™ conform to current EC Medical Devices Directive (93/42/EEC Annex V).
Toe-niquets™ conform to current FDA Directives.
Toe-niquets™ are CE Sterile and carry the CE Class 1 – Sterile Mark.
Toe-niquets™ are FDA Sterile registered and carry a FDA Registered Mark.
Toe-niquets™ meet the criteria and recommendations set out in the UK NHS National Patient Safety Agency's, Rapid Response Report (NPSA/2007/RRR007 dated on 9th December, 2009) aimed at 'Reducing risks of tourniquets left on after finger and toe surgery'

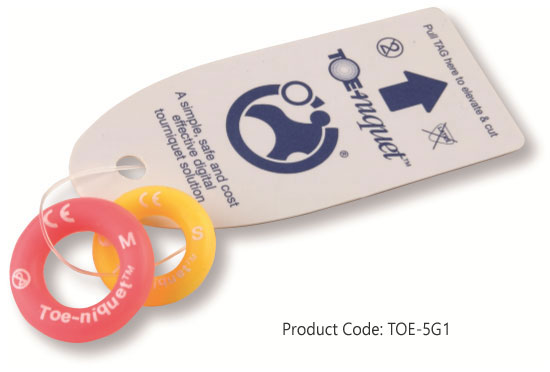
Product Code: TOE-5G1
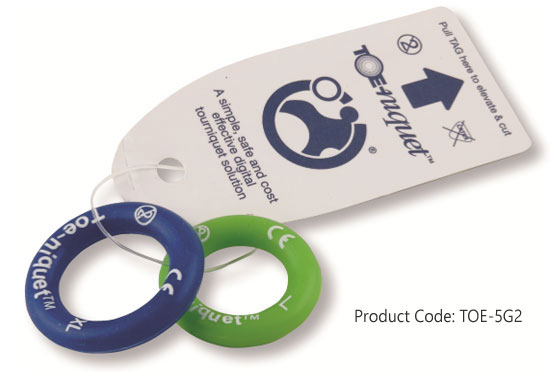
Product Code: TOE-5G2
AneticAid Limited,Leeds, Yorkshire, LS20 9JE (UK)
+44 (0) 1943 878647
sales@aneticaid.co.uk
Please fill the form with your information ... We love to help you